How To Draw Atomic Structure Of First 20 Elements
Atoms (Basic Chemistry Tutorial 1) focused on the general structure of atoms. Here, you'll learn how to determine an atom's specific structure, which will set us up for learning about chemical bonds in the next tutorials.
If you've already watched the video, Click here , or scroll down below the video to start interacting.
1. Drawing the Simplest Atoms: Hydrogen and Helium
The most common element in the universe is hydrogen, a gas which makes up about 99% of the universe's known mass1. Hydrogen is the main component of stars, and a star is, by far the most massive thing in any solar system.
1 That's not including "dark matter," which is beyond the scope of this course.Hydrogen atoms have one proton, and one electron. So we can represent hydrogen like this:
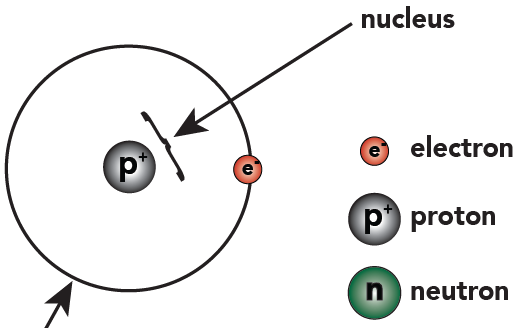
Hydrogen has one single proton that goes in the nucleus. Outside the nucleus orbits a single electron, which is found in the first energy level.
Make sure you've clicked "show the answer" and studied the diagram above before proceeding.
2. The Octet Rule
For atoms with more than two protons and two electrons, a few rules need to be introduced in order to diagram them in a way that represents their chemical properties.1
1When you learn about atoms in a chemistry class, you'll learn a much more sophisticated and accurate model of electron arrangement. But for the biology that you'll learn in an introductory high school (or even college) course, the rules that follow will work.The first rule is called the Octet Rule. "Octet" refers to eight electrons, as you'll see below. The key parts of the rule are:
- Each of the energy levels where electrons are found has a fixed capacity to hold electrons.
- The first energy level can hold up to two electrons.
- The second and third energy levels can hold up to eight electrons (hence, "Octet").
- When one energy level is filled, you start filling the next one.
Let's use the Octet Rule to draw a lithium atom.
Make sure you've clicked "show the answer" and studied the diagram above before proceeding.
From this point on we're going to simplify our diagrams by drawing the nucleus differently.
- Instead of showing individual protons and neutrons, draw a circle to indicate the nucleus.
- Write the number of protons and neutrons inside the circle.
Below on the left, you can see this new representation of lithium, next to the one we've previously used.
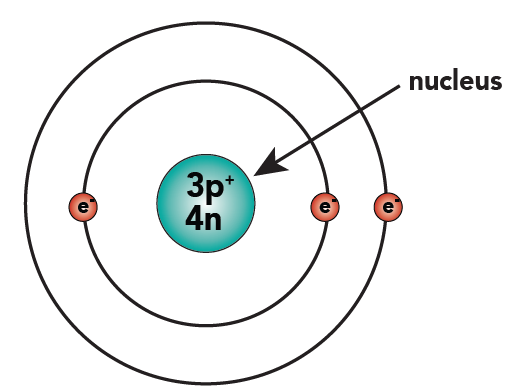 | 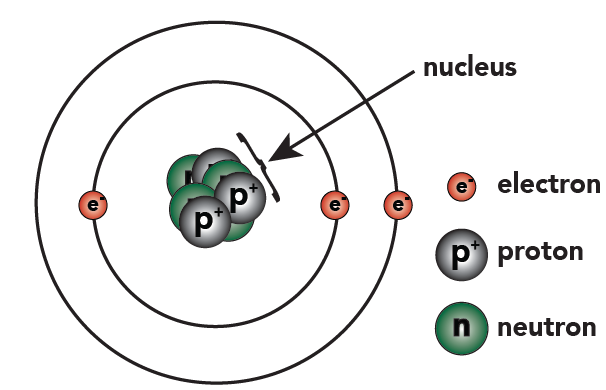 |
| New representation of lithium (notice how protons and neutrons are indicated in the nucleus) | Previous representation of lithium showing individual protons and neutrons |
To represent the atoms of the next few elements, we need one more rule: place the electrons in the energy level singly until you have four, and then pair them up until you have filled the energy level with 8 electrons. As we'll see, this rule will help you to understand what's happening when we learn about chemical bonding, where electrons trade or share electrons. Following that rule, we can draw the next atoms, all the way through neon.
Make sure you've studied the answer to the problem above before going on.
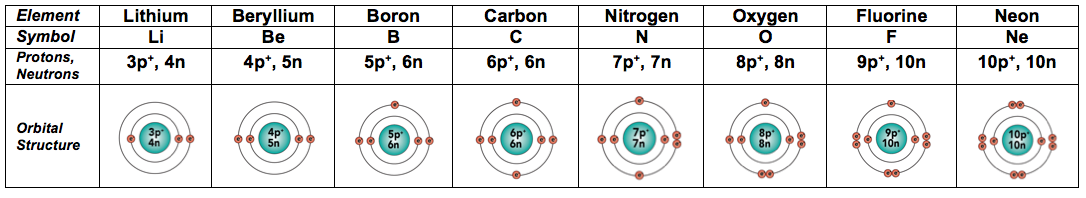
2a. Elements 3 through 10 (Lithium through Neon)
The table below shows all the atoms in the second row of the Period Table of the Elements. Study it and make sure that the arrangement of electrons makes sense to you before going on.
2b. Elements 11 through 20
If the number of electrons in an atom exceeds 10 (the limit of the first two energy levels), then the next electrons go into the third energy level. The rules for filling the third energy level are exactly the same as those described above.
Here are elements 10 through 18, which are the ones in the third row of the Periodic Table. Make sure that their electron arrangement makes sense before reading on.
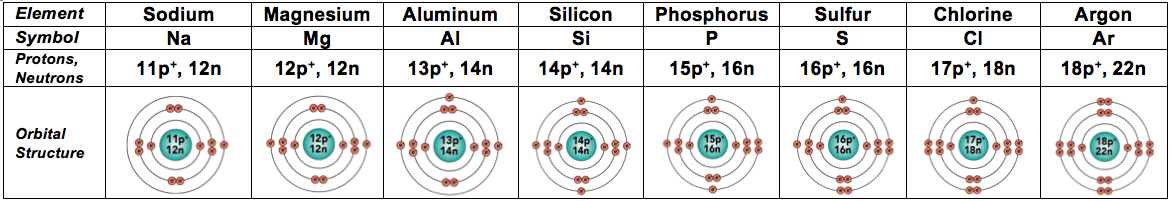
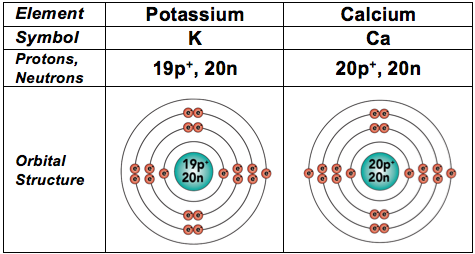
And because they're also fairly common in living things, we'll throw in the next two elements, too. In these elements, electrons start filling the fourth energy level.
3. Review: Four Things to Know about Drawing Atoms
In this tutorial, we've focused on understanding the structure of atoms. We've specifically learned that
- Electrons are found in energy levels outside the nucleus
- The first energy level has a capacity of two electrons
- The next two energy levels each have a capacity of 8 electrons.
- These rules about electron capacity are known as the Octet rule.
Using these rules, if you're given the number of protons that an atom has, you should be able to figure out the arrangement of its electrons.
4. Checking Understanding (Flashcards: Atomic Structure)
To check your understanding, we're going to start with a few flashcards, then move on to an interactive quiz.
5. Quiz: Structure of Atoms
This quiz tests you on all of the vocabulary you've learned so far. It also tests you on your ability to correctly identify an atom's structure.
6. Next steps
If you need more practice, please scroll up to the top and work through this tutorial again. Otherwise, follow the links below:
-
Elements, Compounds, and Molecules (the next tutorial in this series)
-
Chemistry Tutorials Menu (for links to all chemistry tutorials)
How To Draw Atomic Structure Of First 20 Elements
Source: https://learn-biology.com/basic-chemistry-tutorial-2-drawing-atoms/
Posted by: kussreearly.blogspot.com

0 Response to "How To Draw Atomic Structure Of First 20 Elements"
Post a Comment